
Table of Contents
What documents do you need to outline for lab management system Accreditation under ISO/IEC17025:2017 and a summary of ISO/IEC17025:2017 Requirements:
Introduction
Accreditation under ISO/IEC 17025:2017 is primarily concerned with the capability of the labs in terms of customer requirement and lab personnel, lab equipment, and lab test performance rather than only Compliance with the quality management system requirements of the standard.
This is a useful outline to use as a reference if you’re going to prepare a laboratory management system for either a testing laboratory or calibration laboratory or sampling laboratory in accordance with ISO/IEC 17025:2017 Standard.
It will help you to meet documents requirements and to ensure that you designed management system must comply with ISO/IEC 17025:2017.The extremely difficult ISO/IEC 17025:2017 is an accreditation standard instead of a certification standard. We can say accreditation is basically a part of any organization which involve in quality control activities i.e. testing/ calibration lab, R&D lab, Sampling Lab and certification is of whole organization which is performing multiple activities.
Standard requirements are one of the most difficult aspects of setting up a laboratory management system either a testing or calibration laboratory or sampling lab. It’s really overwhelming to read the ISO/IEC 17025:2017 Standard for the first time. After reading the handbook, it seems as you are likely to forget the steps that you might to take or follow for designing a smart management system.
I have designed a plan that you might follow, based on my personnel knowledge and experience while applying to get ISO 17025:2017 Accreditation. Even if there is a set guide ISO/IEC 17025: 2017 for laboratories that we may study and start preparing the documents for its implementation!
You can design your quality manual for your quality management system for either testing or calibration laboratory. Management system is a framework which you should follow while defining appropriate management system including a set of standard procedures forms for of data recording, and other forms & formats required complying with the general requirements of ISO/IEC 17025:2017.
For creating a record that included designing of appropriate form under each procedure for compliance with ISO/IEC 17025:2017 is outline here.
Every lab has a different design for documenting how the ISO 17025:2017 criteria are Implemented, therefore, these requirements aren’t entirely complete. Nevertheless, having essential procedures is an excellent place to start for accreditation of laboratory management system that is in line with ISO/IEC 17025:2017.The following framework can be copied and used to develop an ISO/IEC 17025:2017 testing.Audit checklist. Please feel free to comment on how you would like improvements.

Quality Management System Requirements as per ISO/IEC 17025: 2017
1-Scope
Define a document of make it a part of quality manual.
★Work’s scope (scope of accreditation) for ISO/IEC 17025:2017
You need to define your laboratory scope either you are a testing laboratory and for what kind of testing you are going to apply for i.e. electrical testing, electronic testing, mechanical testing, civil structure test, chemical testing, microbiological testing, agriculture testing, pharmaceutical testing, drug testing, etc. For what test you need to get accreditation. Same as it can be for calibration and sampling laboratory.
2-Normative Reference
★ Define a document of make it a part of quality manual.General vocabulary which is used from ISO/IEC guide 99 and ISO/IEC 17000 series.
3-Terms and Definition
As defined in the ISO/IEC 17025: 2017 standard

4-General Requirements for ISO/IEC 17025:2017
★ Impartiality and confidentiality Procedure
★Supporting documents for procedure i.e.
- Confidentiality Acceptance form,
- Agreement on impartiality
5-Structural requirements for ISO/IEC 17025:2017
- Legal Records (evidence of registrations)
- Chart of the Organization
- Authorizations List
- Staff Appointment letters
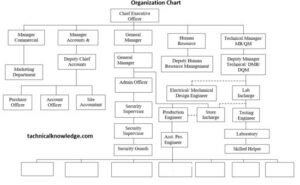
Chart of the Organization
6-Resource Requirements for ISO/IEC 17025:2017
★ Procedure for Personnel
★Supporting documents for procedure i.e.
- Training calendar
- Induction Training Form
- Training report
- Technical training effectiveness evaluation
- Skill matrix or competency
- Authorization/ Appointment Letter
- Responsibilities List and Acceptance
- Job description List and Acceptance
- Qualifications/competency Report
- List of competence requirements
★ Procedure for Facilities and environmental conditions
★Supporting documents for procedure i.e.
- Environmental conduction monitoring from
- Environmental Control chart
- Security Procedure
- Safety procedure
- Housekeeping Checklist
★ Procedure for Equipment’s
★Supporting documents for procedure i.e.
- Equipment Master list
- Preventive maintenance plan
- Details and calibration status of equipment form
- Calibration Plan
- Control chart Statistical techniques Report
- Intermediate check producer
- Intermediate check form
- Preventive maintenance plan
- Preventive maintenance records
- Equipment History Card
★ Procedure for Metrological Traceability
★ Supporting documents for procedure i.e.
- Traceability information Metrological Traceability
- Traceability chart
★ Procedure for externally provided products and services
★Supporting documents for procedure i.e.
- Lab Demand/ Purchase Form
- List of approved suppliers
- Supplier Evaluation & Re-Evaluation Form
- Demand/ Purchase Request Form
- Purchase order
- Inspection form for Incoming Critical Supplies/Equipment
7-Process requirements for ISO/IEC 17025:2017
★ Procedure for Review of requests, tenders and contracts
★Supporting documents for procedure i.e.
- Customer Request Form
- Request details/ Tag
- Certificate Receive form
★ Procedure for Selection and verification methods
★Supporting documents for procedure i.e.
- Verification form
★ Procedure for validation of methods
★ Supporting documents for procedure i.e.
- Validation Form
★ Procedure for Sampling
★ Supporting documents for procedure i.e.
- Sampling plan
- Sampling criteria
- Sampling Record
★ Procedure for Handling of test or calibration items
★ Supporting documents for procedure i.e.
- Handling, Transport, and Storage Procedure
- Test request Tag
- Sample Receiving and Releasing Procedure
- Incoming and Outgoing register
- Receiving Record
★ Technical records
- Technical record details
★ Procedure for Evaluation of measurement uncertainty
★ Supporting documents for procedure i.e.
- Accomplished Measurement Data Sheets
- Calibration certificates
- Measurement uncertainty calculator
★ Procedure for ensuring the validity of results
★ Supporting documents for procedure i.e.
- PT/ ILC results
- PT/ ILC Plan
- PT/ ILC Analysis report
- Functionality check form
- Control Charts of Equipment under use
- Repeatability and Reproducibility Results
★ Procedure for Reporting of results
★ Supporting documents for procedure i.e.
- Raw data sheets
- Test Reports
★ Procedure for Complaints
★ Supporting documents for procedure i.e.
- Complaints Form
- Complaint receiving form
- Complaint Closure form
★ Procedure for Nonconforming work
★ Supporting documents for procedure i.e.
- Non-conforming work reporting form
- Hold and release tag
- Service Report Form
★ Procedure for Control of data and information management
★ Supporting documents for procedure i.e.
- Software Validation record
8- Management Systems Requirements for ISO/IEC 17025:2017
Options for laboratories
★ Procedure for Management system Documentation
★Supporting documents for procedure i.e.
- Lab Policies
- Lab documents record
- Operating instructions/work instructions list
- Documents distribution sheet
★ Procedure for Control of Management system Documentation
★Supporting documents for procedure i.e.
- Lab record sheet
★Procedure for Control of Records
★Supporting documents for procedure i.e.
- Lab record sheet
- Record backup sheet
★ Procedure for Actions to address risk and opportunities
★Supporting documents for procedure i.e.
★ Supporting documents for procedure i.e.
- Risk assessment Matrix
- External and Internal Risk and Opportunity for Improvement Form
★ Procedure for Improvements
★Supporting documents for procedure i.e.
- Customer feedback form
- Improvement measurement form
★ Procedure for Corrective Actions
★Supporting documents for procedure i.e.
- Corrective action form
★ Procedure for Internal Audits
★Supporting documents for procedure i.e.
- Internal audit attendance
- Internal audit checklist
- Internal audit report
- Internal auditor list
★ Procedure for Management reviews
★Supporting documents for procedure i.e.
- Management review attendance
- Management review minutes
- Output of MR meetings
Other Records and Forms which are used depending on the Laboratory scope of work.
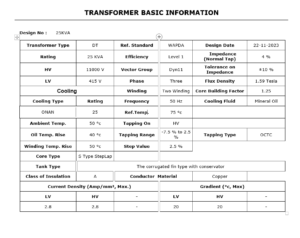
25KVA Distribution Transformer Design pdf
Introduction to Distribution Transformers Design Distribution Transformer Design plays a vital role in electrical power transmission and distribution systems. Among various transformer ratings, the 25KVA

Tunnel Lighting Design And Requirements
1. Introduction of Tunnel Lighting Design Tunnel lighting is critical to ensuring the safety, visibility, and efficient operation of road and transportation tunnels. The design
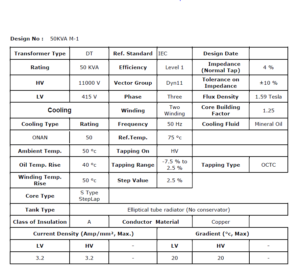
50 KVA Transformer Design complete calculation in Pdf
50 KVA transformer design complete calculation involves a comprehensive PDF sheet of calculations and considerations to ensure its efficiency, reliability, and adherence to safety standards.

What is a Single phase Transformer Construction and Its Working
Transformers, the unsung heroes of our electrical infrastructure, play a pivotal role in the seamless distribution of power. Among these, single-phase transformers stand out for
Unveiling the Secrets of Mastering Start Stop Motor Control Circuits
Do you want to learn more about mastering the Start Stop Motor Control Circuits? You can learn all the details about this vital part of

What is HiPot testing or Dielectric Withstand testing?
1.0 Introduction to HiPot Testing or Dielectric Withstand Testing HiPot testing, short for High Potential Testing, is a critical procedure used in the realm of

Leave a Reply